Living in the tropics, surrounded by jungle-covered hills, corals, picture-perfect beaches and cocktails sounds like a dream. Imagine listening the gentle music of the waves, bird songs, or the characteristic ‘zzzzzzzzzz’ of mosquitoes while enjoying a cup of coffee at the sun set. Walk along the beach, go for a swim to cool off from the sun’s warmth and do some snorkeling. This is paradise.
This is a dream that comes true for testate amoebae.
Travelling through tropical countries is such an experience that most people dream of returning; the same holds true for testate amoebae. Visiting tropics is one thing but by actually accumulating down some shells, testate amoebae gained a much better perspective on just how awesome the warmer part of the world is compared to the cold and dark side of the world. With the sun, sand and sea at your pseudostome-door and a relaxed way of life, the tropics has it all.
Tropics are so diverse that testate amoebae can enjoy various and nice places to live in. Sphagnum mosses in the Galapagos is an obvious spot to enjoy the tropics (Fournier et al. 2015), but why always live in a moss while you can experience other habitats? Living in the tropics also means sometimes living differently from what you already know. In such purpose, the jungle seems appropriate! The jungle is a great place to go hiking and trekking, and incidentally see how diverse and valuable these ecosystems are. Plus, it’s usually the best place to spot new habitats, particularly if you are an amoebae. Pseudopoding through the dense and muddy vegetation gives a sense of true exploration, you never know what you’ll find. That’s what happened to testate amoebae when they ended up in tank-bromeliads as they wanted to cool off after a hike through the humid jungle.
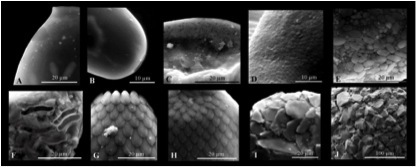
Bromeliads include mainly epiphytic rosette plants occurring mostly in Central and South America. They collect rain water and particulate materials in tanks (cisterns) formed by the coalescing leaf axils. These tanks form micro-ecosystems above the ground where groups of freshwater organisms ranging from algae, fungi, bacteria and protozoa through insects to frogs are represented and constitute considerable populations (Frank 1983, Laessle 1961, Maguire 1971, Picado 1913, Richardson 1999). Of special interest to us, these tanks are inhabited by many characteristic protists and small metazoans (Picado 1913; Maguire 1971; Martinelli 2000; Schönborn 2003). The only way for Nebela tincta, a widely distributed species, to meet Polyplaca globosa (Figure 1), P. monocornica and P. invaginata is to visit a Brazilian tank-bromeliad (Torres-Stolzenberg 2000).

Figure 1: Polyplaca globosa; an endemic testate amoebae from tank-bromeliads (Picture from Torres and Schwarzbold, 2000)
The vibe is unbelievable in the tropics! The common view is that no one seems to be in a hurry for anything. Such a cliché is obviously false; this includes testate amoebae in tank-bromeliads. Life in tank-bromeliads is not so quiet but rather dangerous. The aquatic food web inhabiting tank-bromeliads consists of micro- and macroinvertebrates (Kitching 2000) and microorganisms such as bacteria, algae, flagellates, fungi and protozoa (Carrias et al. 2001). While testate amoebae can be fearsome predators (Gilbert et al. 2000, Geisen et al. 2015) in mosses and soils, they are neither the largest nor the most dangerous in tank-bromeliads (Carrias et al. 2001). Micro- and macroinvertebrates can be very interested to feed on testate amoebae, and when you’re trapped in a tank, your chance to escape a predator are very low. This may explain why testate amoebae abundance is so low in tank-bromeliads compared to other protists such as ciliates (Carrias et al. 2012).
Tank-bromeliads are fascinating to me. Recent studies showed that microbial communities in bromeliads are highly distinct from the surrounding environments, for example soil, with sometimes a strong shift towards crucial ecosystem functions such as methanogens (Goffredi et al. 2011a, b; Mattinson et al. 2010, Louca et al. 2016). In temperate and subarctic ecosystems, testate amoebae are abundant and suggested to be key in ecosystem processes such as decomposition (Lamentowicz et al. 2013; Geisen et al. 2015; Jassey et al. 2016) or carbon uptake (Jassey et al. 2015). The low abundance of testate amoebae in tank-bromeliads suggest either they are not key species in microbial interactions and connected ecosystem processes, or they are a key trophic-link between microorganisms and micro- and macro-invertebrates as preferential food resource.
Clearly, more studies focusing on the role of testate amoebae in tank-bromeliads are needed. There are so many reasons to go and live in the tropics. If you need a good excuse to go in the tropics and see how living there is awesome, I hope this post will give you a good one. For the others who hate warm temperatures, corals, picture-perfect beaches, and rather love winters, but who would like to study testate amoebae in tank-bromeliads, I heard that some testate amoebae found an alternative to the tropics by living in bromeliads…from florist wholesalers (Kolicka et al. 2016).
References
Carrias et al. A preliminary study of freshwater protozoa in tank bromeliads. Journal of Tropical Ecology, 17, 611–617 (2001).
Carrias et al. An ant–plant mutualism induces shifts in the protist community structure of a tank-bromeliad. Basic and Applied Ecology. 13, 698–705 (2012).
Fournier et al. A legacy of human‐induced ecosystem changes: spatial processes drive the taxonomic and functional diversities of testate amoebae in Sphagnum peatlands of the Galápagos. Journal of Biogeography 43, 533-543 (2015).
Frank. Bromeliad phytotelmata and their biota, especially mosquitoes. Pp. 101–128 in Frank, J. H. & Lounibos, L. P. (eds). Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Plexus Publishing Inc., Medford (1983).
Goffredi, Kantor & Woodside. Aquatic microbial habitats within a neotropical rainforest: bromeliads and pHassociated trends in bacterial diversity and composition. Microbial Ecology 61, 529–542 (2011a).
Goffredi et al. Bromeliad catchments as habitats for methanogenesis in tropical rainforest canopies. Frontiers in Microbiology 2, 256 (2011b).
Jassey et al.. An unexpected role for mixotrophs in the response of peatland carbon cycling to climate warming. Scientific Reports, 1–10 (2015).
Jassey et al.. Loss of testate amoeba functional diversity with increasing frost intensity across a continental gradient reduces microbial activity in peatlands. European Journal of Protistology, 55, 190–202 (2016).
Kitching. Food Webs and Container Habitats: The Natural History and Ecology of Phytotelmata. Cambridge University Press, Cambridge (2000).
Kolicka et al. Hidden invertebrate diversity – Phytotelmata in Bromeliaceae from palm houses and florist wholesalers (Poland). Biologia 71, 194-203 (2016).
Laessle. A micro-limnological study of Jamaican bromeliads. Ecology 42:499–517 (1961)
Louca et al. High taxonomic variability despite stable functional structure across microbial communities. Nature Ecology & Evolution, 1, 1–12. (2016).
Maguire. Phytotelmata: biota and community structure determination in plant-held waters. Annual Review of Ecology and Systematics 2:439–464 (1971).
Martinelli. Gefährdete Raritäten. Bromelien im at- lantischen Regenwald. Spektrum der Wissenschaft 6/2000, 66–73 (2000).
Martinson et al. Methane emissions from tank bromeliads in neotropical forests. Nature Geoscience 3, 766–769 (2010).
Picado. Les Broméliacées epiphytes considérées comme milieu biologique. Bulletin Scientifique de la France et de la Belgique 47:215–360 (1913).
Richardson. The bromeliad microcosm and the assessment of faunal diversity in a neotropical forest. Biotropica 31:321–336 (1999).
Schönborn. Lehrbuch der Limnologie. Schweitzerbart, Stuttgart (2003).
Torres and Schwarzbold. Procta em associacao com Vriesea sp. (Bromeliaceae): tres novos taxa de amebas tes- taceas (Protoctista: Rhizopoda, Testacealobosea). Not. Faun. Gembloux 41, 105–113 (2000).